ABout Us
The Company
Sigachi has always been driven by an aspiration to deliver significant value to all stakeholders. After being a market leader in MCC Excipients, Sigachi now has diversified offerings – Active Pharmaceutical Ingredients (APIs), Food and Nutrition, Operations & Management, and the Personal Care industry. Sigachi’s advanced manufacturing facilities are built with a reliable and efficient supply chain. It’s Government of India-approved R&D Laboratory and state-of-the-art Excipient Application Lab, harnesses it’s innovation and technical excellence.

Sigachi is attuned to the highest standards of quality. All five of its multi-location facilities hold esteemed certifications, including EXCiPACT GMP, SGMP, HACCP, EDQM CEP, FSSAI, USFDA, and ISO 9001:2015, highlighting the company's non-negotiable commitment to safety, quality, and regulatory excellence. Sigachi has built a reputation as a reliable partner, with a broad reach spanning India, Asia, Australia, America, Europe, and the Middle East.
Sigachi MENA
Sigachi MENA (SM) serves as a key regional entity headquartered in Dubai, UAE, under the umbrella of Sigachi Industries Limited (SIL). SM oversees two subsidiaries: Sigachi Arabia (SA), based in Riyadh, Saudi Arabia and Sigachi Global, headquartered in Abu Dhabi, UAE.
Sigachi MENA plays a pivotal role in driving business development and geographical expansion across the region.
Sigachi Arabia is strategically positioned to capitalize the growth opportunities in the emerging Saudi market. By closely collaborating with local authorities, SA actively contributes to the “Make in KSA” initiative, aligning with Saudi Arabia’s Vision 2030 to foster self-sufficiency and economic diversification.
Sigachi Values
Excellence
Delivering goods & services of higher standards, thereby creating value and prosperity for all stakeholders.
Respect
Treating everyone with equality and having respect for the environment. Being truthful and building trust through our actions.
Integrity
Being honest in who we are and what we do. We take responsibility of our actions, which includes observing all the laws and regulations and encourage others to do so.
Resourcefulness
We find creative and ingenious solutions, which are within the best interest of Sigachi and its customers. If we own a problem, we don’t sit back.
Our Leadership
Sigachi, incorporated in 1989, is a global leader in manufacturing Microcrystalline Cellulose (MCC) with a diversified presence in pharmaceuticals, food, nutraceuticals, and cosmetics. With five advanced manufacturing facilities across India, Sigachi ensures reliable supply and top-tier quality for its products, exported to over 65 countries. The company is committed to innovation, sustainability, and expanding its global footprint, making it a trusted partner in various industries.

- Board Of Directors
- Management














Our Business
Industries we serve
Pharmaceutical
Our comprehensive range of pharmaceutical excipients is uniquely positioned to deliver integrated solutions for pharmaceutical and nutraceutical manufacturers specializing in oral drug delivery systems. We offer Active Pharmaceutical Ingredients (APIs) for anti-ulcer (gastrointestinal) treatments, along with pre-formulated excipients, high-functionality excipients, thickeners/stabilizers, binders, lubricants, carriers, and superdisintegrants to support diverse formulation needs.

Food & Nutrition
Our additives enhance the freshness and flavor of food products, ensuring they remain delicious for an extended period. Our portfolio for the food industry includes colloidal grades of E 460(i) or MCC, Cellulose Powder, and proprietary premixes under the JoyMix brand. All products are certified to meet stringent standards, including FSSAI, FSSC 22000, ISO, GMP, and other regulatory benchmarks, reflecting our commitment to quality and safety.
Operations & Management
Our Operations & Maintenance (O&M) services are designed to optimize production while prioritizing safety and quality across sectors such as Chlor-Alkali, Petrochemicals, Fertilizers, Solar, Desalination, Utilities, and ETP. We focus on enhancing productivity, reducing waste, and ensuring full compliance with regulatory requirements.
We maintain equipment reliability through regular inspections, preventive maintenance, and condition-based monitoring for effective asset management. Ensure regulatory compliance and operational safety through risk assessments, robust training, and continuous evaluation. Empower employees with essential skills in safety, equipment handling, and compliance to foster innovation and sustain competitive performance.
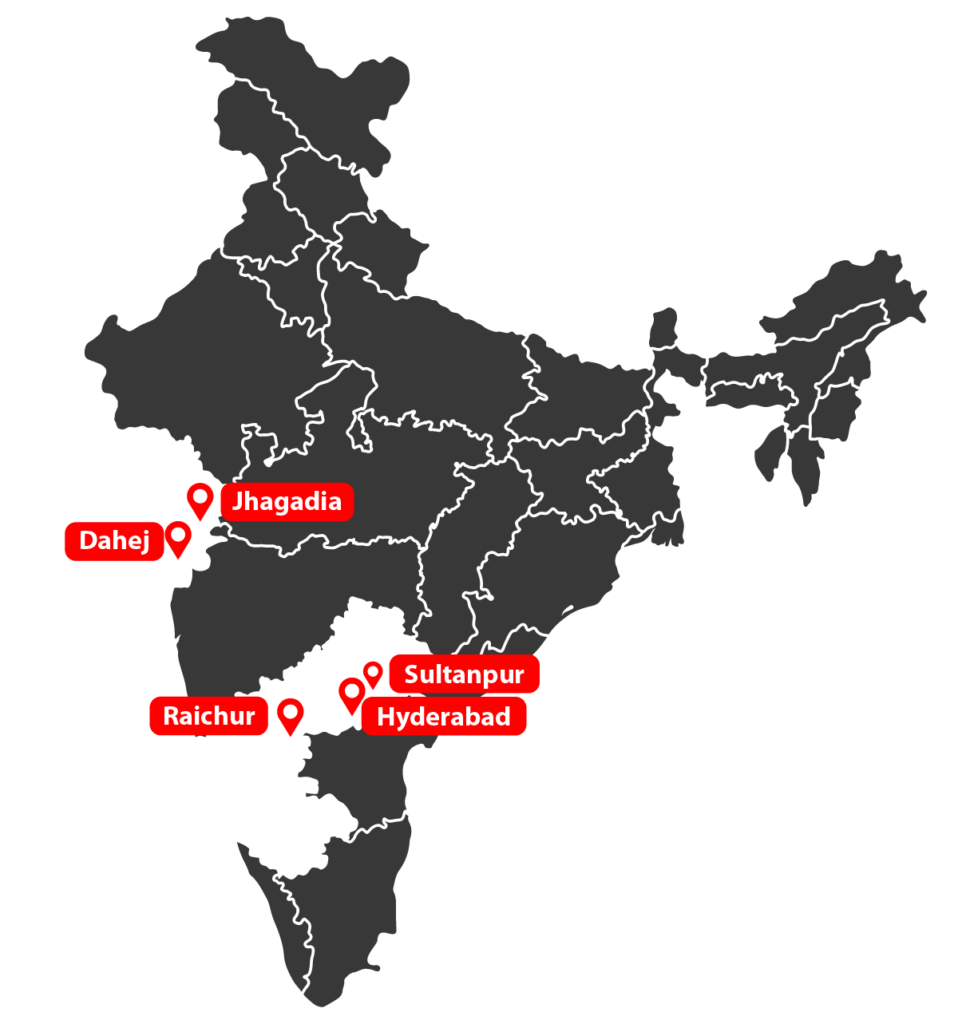
Our facilities
Multilocational facilities in Hyderabad, Sultanpur, Jhagadia, Dahej, Raichur, Telangana, Gujarat, and Karnataka.
Hyderabad Unit -
- One facility is in the Special Economic Zone dedicated to premium exports business and one facility dedicated to nutritional premix manufacturing.
- Two State-of-the-art R & D Application lab facilities- Excipient Application Lab and Food Application lab (approved by Government of India, Ministry of Science & Technology, Department of Science and Industrial Research).
Quality Certification
For over three decades, Sigachi has excelled in understanding evolving customer needs, delivering consistent quality products, and ensuring batch-to-batch uniformity. The quality excellence is reinforced by stringent quality testing of raw materials, in-process checks, and finished products, all supported by a world-class Quality Control laboratory equipped with advanced instruments. The Quality Assurance team conducts regular internal audits, ensuring adherence to GMP (Good Manufacturing Practice) norms and IPEC (International Pharmaceutical Excipients Council) guidelines across sourcing, manufacturing, and packaging processes. The quality and compliance are further validated by essential industry certifications like-









connect with us
Get in touch with our experts
Regional address
Sigachi MENA,
- IFZA properties, Dubai Silicon Oasis, Dubai - UAE
- farook.h@sigachi.com

